
▌Tunable CO2 capture in N-ethylethylenediamine functionalized Mg2-MOF-74: unraveling the role of diamine basicity in reactivity and adsorption capacity
N-乙基乙二胺功能化Mg₂-MOF-74中可調式CO₂捕集:二胺鹼性在反應性與吸附容量中的關鍵角色
Santhanamoorthi Nachimuthu, Mao-Sheng Su, Liang-Ting Wu, Ching-Tsung Yu, Jyh-Chiang Jiang*
https://doi.org/10.1016/j.cej.2025.163587
SEED Member: Jyh-Chiang Jiang
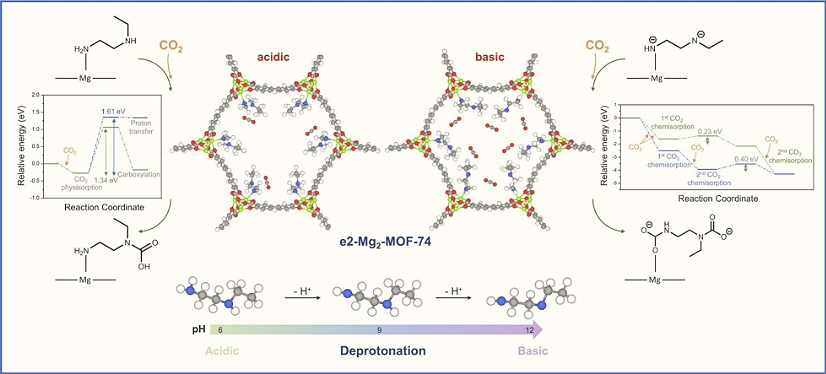
Schematic illustration
Major Contributions
1.This study systematically identifies N-ethylethylenediamine (e2) as an optimal diamine for functionalizing Mg₂-MOF-74, demonstrating that e2 provides both favorable CO₂ adsorption energy and a low energy barrier for the rate-determining step in the capture process, thus enabling efficient and selective CO₂ capture.
2.By elucidating the effect of diamine basicity (pKa) on the adsorption mechanism, reaction barriers, and overall CO₂ uptake, this work reveals that mono- and doubly deprotonated forms of e2 significantly lower the energy barriers for CO₂ insertion at both amine sites, with the doubly deprotonated framework achieving enhanced reactivity and the capacity to capture up to 24 CO₂ molecules.
3.The research establishes that a solvent environment with a pH of approximately 12 favors the formation of the doubly deprotonated e2 species, which optimizes CO₂ adsorption performance and facilitates efficient material regeneration, providing theoretical guidance for the design of advanced CO₂ capture materials.
主要貢獻
1.本研究系統性地篩選出N-乙基乙二胺(e2)作為功能化Mg₂-MOF-74的最佳二胺,證明e2具有優異的CO₂吸附能與低能障,有效提升CO₂捕集的效率與選擇性。
2.透過闡明二胺鹼性(pKa)對吸附機制、反應能障及整體CO₂吸附量的影響,發現e2的單去質子與雙去質子狀態能顯著降低CO₂插入兩個胺位點的能障,尤其雙去質子態的材料展現出更高反應活性,CO₂吸附量可達24分子。
3.研究證實,溶液pH約為12時,有利於雙去質子e2物種的形成,進而最佳化CO₂吸附表現並促進材料再生,為先進CO₂捕集材料的設計提供理論依據。


