
▌Li–Sb Alloy Formation Strategy to Improve Interfacial Stability of All-Solid-State Lithium Batteries
鋰銻合金形成策略以提升全固態鋰電池界面穩定性
B.D. Dandena, W.N. Su*, D.S. Tsai, Y. Nikodimos, B.W. Taklu, H.K. Bezabh, G.B. Desta, S.C. Yang, K. Lakshmanan, H.S. Sheu, C.H. Wang, S.H. Wu, B.J. Hwang*
https://doi.org/10.1002/smtd.202400571
SEED Member: W.N. Su, Y. Nikodimos, S.C. Yang, S.H. Wu, B.J. Hwang
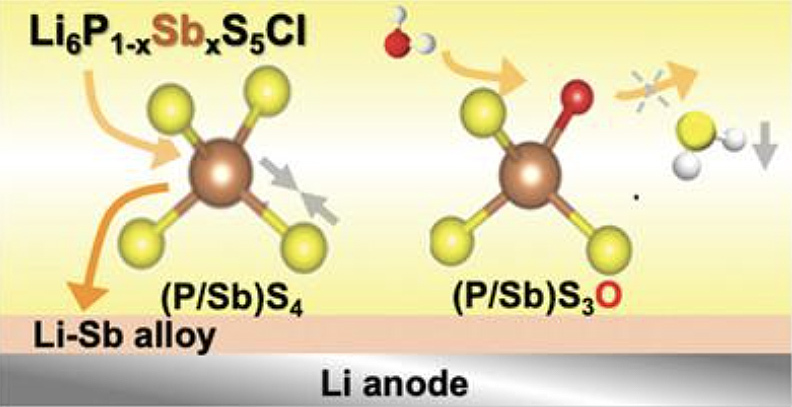
Major Contributions
1. Development of a novel Li-Sb alloy formation strategy through antimony doping in lithium argyrodite solid electrolyte (Li6PS5Cl), achieving the highest critical current density of 14.5 mA cm-2 among reported sulfide solid electrolytes without lithium dendrite penetration.
2. Successful synthesis of Sb-doped Li-argyrodite (Li6P1-xSbxS5Cl) electrolyte demonstrating superior ionic conductivity (9.206 mS cm-1) at room temperature and exceptional cycling stability (1900h at 0.1 mA cm-2 and 900h at 1.0 mA cm-2).
3. Enhancement of air stability through Sb substitution, resulting in minimal H2S generation (only 0.7 cm3 g-1) after 30 minutes exposure to 20% humid air, attributed to the strong Sb-S bonding compared to P-S bonding.
主要貢獻
1. 開發新穎的鋰銻合金形成策略,通過在鋰銀紅石固態電解質(Li6PS5Cl)中摻雜銻,實現了硫化物固態電解質中最高的臨界電流密度14.5 mA cm-2,且無鋰枝晶穿透。
2. 成功合成銻摻雜鋰銀紅石(Li6P1-xSbxS5Cl)電解質,展現出優異的室溫離子電導率(9.206 mS cm-1)和卓越的循環穩定性(在0.1 mA cm-2下可維持1900小時,在1.0 mA cm-2下可維持900小時)。
3. 通過銻取代提升空氣穩定性,暴露在20%濕度空氣中30分鐘後僅產生0.7 cm3 g-1的H2S,這歸因於Sb-S鍵比P-S鍵更強的特性。


