
▌Fundamental phenomena in anode-free coin cells and pouch cells configured with imide salt-based ether electrolytes
以醚類電解質搭配亞醯胺鹽之無陽極鈕扣電池與軟包電池的基礎現象研究
K.N. Shitaw, M.A. Weret, Y. Nikodimos, T.M. Tekaligne, S.K. Jiang, C.J. Huang, B.H. Lin, S.H. Wu, W.N. Su*, B.J. Hwang*
https://doi.org/10.1016/j.mtener.2023.101461
SEED Member: S.H. Wu, Y. Nikodimos, S.C. Yang, W.N. Su*, B.J. Hwang*
Major Contributions
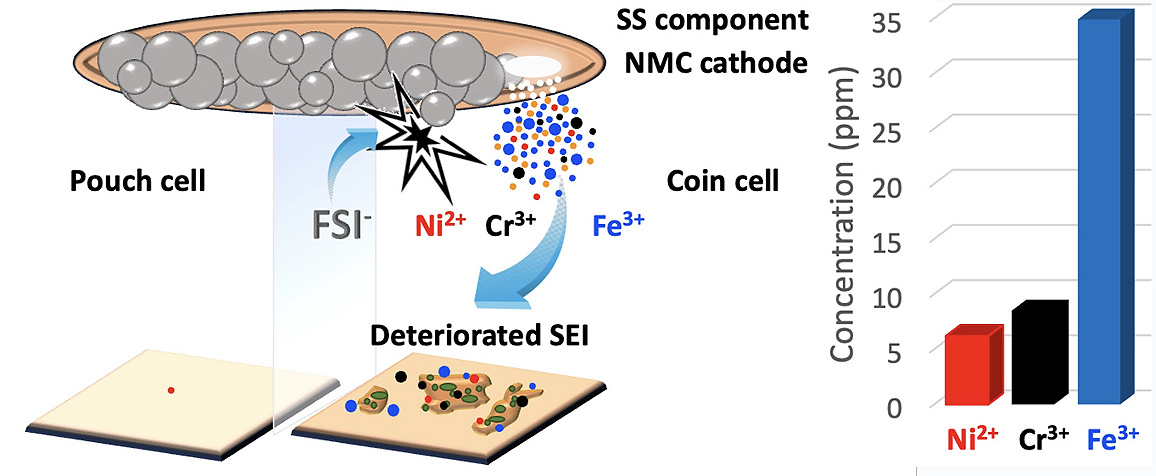
1. Discovered and explained the significant performance discrepancy between coin cells and pouch cells in anode-free lithium metal batteries using imide salt-based ether electrolytes, particularly identifying that coin cell corrosion leads to severe capacity fading.
2. Systematically investigated and quantified the cross-talk effects of transition metals (Ni, Cr, Fe) at the anode side, revealing that coin cells suffer from significant metal accumulation (6.2, 8.5, and 34.9 ppm respectively) after 15 cycles, leading to >10.4% irreversible coulombic efficiency.
3. Demonstrated that pouch cells achieve superior performance with 99.1% average coulombic efficiency after 120 cycles and minimal metal contamination (~0.3 ppm Ni), establishing pouch cells as a more reliable configuration for evaluating ether electrolytes in anode-free battery systems.
主要貢獻
1. 發現並解釋了在使用亞醯胺鹽基醚類電解質的無陽極鋰金屬電池中,鈕扣電池和軟包電池之間存在顯著的性能差異,特別指出鈕扣電池的腐蝕會導致嚴重的容量衰減。
2. 系統性研究並量化了過渡金屬(鎳、鉻、鐵)在陽極側的串擾效應,揭示鈕扣電池在15次循環後出現顯著的金屬累積(分別為6.2、8.5和34.9 ppm),導致超過10.4%的不可逆庫倫效率。
3. 證實軟包電池可實現更優異的性能,在120次循環後達到99.1%的平均庫倫效率,且金屬污染極低(僅約0.3 ppm鎳),確立軟包電池作為評估無陽極電池系統中醚類電解質的更可靠配置方案。


